Vaccines hit the headlines, while Paxlovid keeps scoring points. Surges, guidelines, Omicron tidbits, herd immunity, excess mortality, and proof that conspiracy theories are harder to kill than zombies.
Treatment news
 |
| A lithium mine in Nigeria |
Lithium carbonate: Yes, someone has given this classic psychiatric drug to 15 hospitalized patients. They were discharged twice as fast as a control group, but it wasn’t statistically significant due to small numbers. Many drugs that seem beneficial in tiny, open-label studies don’t hold up in larger trials, and I’d guess this will be one of them.
Paxlovid:
Efficacy: A preprinted Israeli study answers two important hitherto open questions: Is Paxlovid effective against Omicron? Does it work in people who are vaccinated? Yes, and yes. In people over 65 its efficacy against Omicron was 67% overall, not far from the 85-88% against Delta. It was 60% in older patients previously vaccinated or infected, 82% in those with no prior immunity. It didn’t help younger high-risk patients, especially those with some prior immunity; the apparent value in those without it is based on few cases and is statistically non-significant.
Symptom issues: Should people who test positive without symptoms take Paxlovid? I’ve heard that some of my colleagues suggest it, though the Phase 3 trials enrolled only people with clinical covid. CDC guidelines are slightly ambiguous. On the one hand: “If you test positive and are more likely to get very sick from COVID-19, treatments are available that can reduce your chances of being hospitalized or dying,” as though maybe that includes the asymptomatic …on the other hand: “It should be started as soon as possible and must begin within 5 days of when your symptoms start.” The FDA says firmly that Paxlovid is authorized “For the treatment of mild-to-moderate COVID-19.” I stand with the FDA, and others: don’t use Paxlovid or remdesivir unless you are ill.
Average-risk patients: Early reports from Pfizer suggested that Paxlovid was 70% effective in patients without major risk factors. But now the company admits that those results have failed to hold up. Standard-risk patients don’t heal faster, and their risk of hospitalization falls only by 50%, non-significant because so few low-risk patients went south (5 vs. 10).
“Rebound”: A study of 483 high-risk patients treated with Paxlovid seems to have found that the treatment failed in 2 patients who went on to require hospitalization, and that 4 others, all boosted, had milder relapses. I say “seems” because contrary to the usual pandemic practice this article is hidden behind a firewall. The summary at the physicians-only MedPage Today website is unhelpful – I can’t even figure out whether there were 4 relapses or 6. And the summary specifies oddly and somewhat ominously: “Patients who failed to complete the 5-day course, lacked significant improvement in symptoms, or had persistent symptoms indicating long COVID were excluded.”
Treatment eligibility: Criteria for outpatient antivirals and sotrovimab are extremely restrictive in the UK(immunosuppression or very severe comorbidities). In the US, on the contrary, almost everybody over 50 is eligible (though the drug is really only useful in the elderly). In practice well-off, well-educated Americans are much more likely to receive treatment, though steps are being taken to improve access for the masses. In Italy those criteria were until recently as rigid as in the UK. So as of late May only 12,000 outpatients had received Paxlovid and 10,000 remdesivir, though about 2.5 million people over 65 had contracted COVID-19 since those antivirals were approved. The much less desirable molnupiravir had gone to 25,000 patients, and the anti-Omicron monoclonal antibody sotrovimab to zero. But I’m pleased to report that as of June 1st Italy has broadened those criteria considerably, making anyone over 65, and younger people with obesity, uncontrolled diabetes, chronic lung disease, etc., eligible for Paxlovid and remdesivir. And National Health Service GPs can now prescribe Paxlovid, though not all pharmacies carry it.
Convalescent plasma: Immunocompromised patients respond poorly to remdesivir and are often taking medications incompatible with Paxlovid, but they’re giving convalescent plasma something of a comeback. Not only has test tube research shown that its polyclonal antibodies can neutralize even the variants that didn’t infect the donor, but there is direct evidence that high-dose plasma saves lives in hospitalized immunodepressed patients.
Monoclonal antibodies: I’ve never understood why Evusheld, AstraZeneca’s combination of tixagevimab and cilgavimab, was being pushed solely for prevention, not treatment. Now researchers report having tested it in unvaccinated patients with early mild-to-moderate COVID-19. It was only modestly effective, cutting progression by 50%. And that was against early strains of SARS-CoV-2, long before Omicron or even Delta. In vitro, though, it seems both bebtelovimab and cilgavimab can effectively neutralize all the Omicron subvariants, including BA.4 and BA.5. Hope they hit the market soon.
Multivalent glycoclusters: Say what? It’s the latest nasal spray hoped to block the SARS-CoV-2 virus from sticking to the lining of the nose and getting people sick. So far it’s only been studied in test tubes – next up: mice.
I-SPY COVID: This multifaceted Phase 2 trial in hospitalized patients has now presented its final results in preliminary form. All 6 tested treatments flunked out: Cenicriviroc, icatibant (Firazyr), apremilast (Otezla), IC14, inhaled dornase (Pulmozyme), and celecoxib (Celebrex) plus famotidine (Pepcid).
Vaccines
Children and teens: Moderna’s trial in 5-to-11-year-olds, using two 50 mcg doses of vaccine, has now been published. Vaccination elicited a strong immune response, and efficacy against clinical COVID-19 during the Delta wave was a very respectable 88% even just 2 weeks after the first dose. It was also 63% effective in preventing asymptomatic infection, with virtually no sign of waning at 3 months. Unfortunately this trial was pre-Omicron, thus less relevant nowadays. Until recently Pfizer has been the only option for anyone under 18, but now the FDA has conceded an Emergency Use Authorization for Moderna in the 5-17 age group, with 12-to-17-year-olds getting the adult 100 µg dose.
Babies and toddlers: Both Pfizer and Moderna now have Emergency Use Authorizations for their vaccines for little kids, and the CDC quickly gave its stamp of approval, so parents of small children will soon have major decisions to make.
First, should they vaccinate their kiddies? Hard to say, since few small children get COVID-19 at all and they rarely become severely ill. A few helpful figures: as of May 11th, 202 Americans between 6 months and 5 years old have died from COVID-19; other sources make it roughly as many as from influenza and pneumonia. Parents whose kids have already had COVID-19 need to note that previous infection, especially during the Omicron era, does not give reliable future protection. I can’t give a strong recommendation for or against.
Second, which vaccine should they prefer, if offered the choice? Pfizer said that 3 doses of its hyper-low dose (3 µg) vaccine had 80% efficacy against Omicron. But that’s absurd, because the so-called efficacy was based on just 10 cases. Moderna’s heftier version using two 25 µg doses has proven only 51% effective against Omicron in children between 6 months and 2 years old, and 37% in 2 to 5-year-olds – more plausible, about what we see in adults. Well over 100 cases had occurred in each age group, making Moderna’s figures much more reliable. Pfizer also has a timing problem: the first two doses don’t even stimulate antibody formation, so kids would be completely unprotected for 3 months after their first shot. I’d personally go for Moderna.
COVID-19 survivors: Israeli research further confirms that hybrid immunity, the combination of SARS-CoV-2 infection and vaccination, gives stronger and longer-lasting protection than either on their own. But Omicron infection in unvaccinated individuals (mice, in this study) seems to protect only against the same subvariant that caused the initial infection, though in vaccinated individuals it intensifies the protection against all variants.
AstraZeneca: A recent CNN piece claimed a New England Journal of Medicine article had shown AstraZeneca protected longer against COVID-19 than Pfizer. Yes and no. AstraZeneca’s effectiveness against infection putatively rose from 58% early on to 70% after 134 days, but those numbers were based on such scanty data that the authors themselves minimized their significance. And we already knew that after 6 months AstraZeneca is utterly useless against Omicron, with Pfizer not much better. I wouldn’t rehabilitate the AstraZeneca vaccine on the basis of this latest study.
 |
| A Covifenz plantation |
Covifenz: The more I think about it, the more puzzled I am that the world’s top medical publication, the New England Journal of Medicine, chose to publish the Phase 3 trial of the Canadian “plant-based” vaccine. I already mentioned the trial’s major weaknesses – enrolling almost nobody over 65 or with comorbidities, and completion before the Omicron surge. But I didn’t notice how much less effective it is not only as compared to the mRNA vaccines but also as compared with the very similar Novavax, until the French expert Benjamin Davido pointed it out (in an interview whose execrable English screams Google Translate). Novavax has 90% overall efficacy, Covifenz just 70%; efficacy against severe disease 100% vs. 78%. And, remember, that’s in working-age adults, usually the group best protected by COVID-19 vaccines.
Novavax: This spike protein vaccine is finally nearing Emergency Use Authorization by the FDA, a mere 15 months after interim Phase 3 results began to trickle in, 10 months after the first article was published, and 6 months since the best trial published results. Apparently the snail’s pace is due to slow vaccine production. Only a handful of doses have been given in the European Union despite authorization, and none of the 1.1 billion doses promised to COVAX have come through. And the FDA briefing document contains other discouraging surprises: Novavax may cause even more myocarditis than the mRNA vaccines, and manufacturing processes vary so much from plant to plant as to raise concerns about safety and efficacy.
Covaxin: After Novavax the next vaccine in the pipeline seems to be this home-grown Indian vaccine, which claimedan overall efficacy of 78% against any COVID-19 and 93% against severe disease. But as I wrote at the time, efficacy was only 66% in people over 60 or otherwise vulnerable, and only 65% against the Delta variant even in the young – all pre-Omicron. Covaxin is an inactivated whole virus vaccine, like Sinopharm and Sinovac, but a more powerful adjuvant supposedly makes it work better. The FDA is making the company do another trial to see whether the vaccine induces as strong an immune response in an American population as it did in the original Indian trials, and apparently plans to award an Emergency Use Authorization if those results come through. Some think a vaccine based on the whole virus rather than just on the spike protein might be better at neutralizing new variants, but given the poor real-life performance of the Chinese whole-virus vaccines I wouldn’t count on it.
Moderna’s new booster: Moderna didn’t do well with a previous attempt at a multivariant vaccine, but now it’s tried again, with a half “regular” and half Omicron-specific booster. According to media reports Moderna has been toutinga 75% improvement in anti-Omicron antibodies over the ordinary Moderna booster as a brilliant result. A leading virologist called this “only a modest benefit,” and questioned whether a broad fall rollout of the new booster would be worthwhile. After reading the detailed preprint, though, I see that the results were actually better than that, with Omicron antibodies more than doubling after 4 weeks, and remaining up there for 6 months. Promising. But antibody levels don’t necessarily translate into real-world protection, and those antibodies were against the original Omicron variant, not against the BA.2 and later subvariants now dominating new infections. With new strains popping up practically every month, who knows how relevant those antibodies are even now, much less this fall?
How to keep efficacy from waning: An immunologist who studies prolonging immunity has penned a New York Times Op-Ed describing what he considers possible approaches: 1) boost with the Johnson & Johnson vaccine, whose protective effect, while relatively low, is relatively steady over time; 2) boost with vaccines like Moderna’s latest, engineered to cover current variants; 3) develop nasal spray vaccines aimed to keep the virus from invading the body; 4) go for universal, all-variant vaccines. The latter option is of course the best, and I was excited to see the author saying universal vaccines have “shown great promise” in animal studies. But the excitement faded when I clicked through and saw he was citing a Times article from February 2021 and, even worse, that the data it featured went back all the way to August 2020. A scientist working actively to develop a universal vaccine estimates a time scale of 2 or 3 years.
Omicron woes
Yes, the Omicron variant usually causes milder disease, but nonetheless it’s already killed as many older Americans as Delta!
The BA.4 and BA.5 subvariants will soon dominate in the US and Europe. They already account for 34% of new cases in Italy and 22% in the USA and are spreading fast, with the vast majority of the rest the very similar BA.2.12.1. Should we worry? No, because there’s no evidence they cause more severe disease. Yes, because they escape preexisting immunity even better than the highly infectious original, infecting even people who’ve had vaccine boosters or have recovered from earlier strains of Omicron.
You may remember that in February the CDC stopped tracking the pandemic with the Community Levels of Transmission that showed how many people were getting sick. The new “Community Levels” sound similar but reflect a very different reality, the percentage of COVID-19 beds occupied in local hospitals. Waving that magic wandartificially slashed the number of Americans living in high-risk counties. A site calling itself the People’s CDC did, however keep following the older, more valid measure and constantly updates an animation tracking the situation. At some point the CDC threw in the towel, adding back maps using the old parameter. Look at how great the US is doing if you just look at hospital capacity (left) and how poorly it’s actually doing (right).
And Congress still hasn’t approved continued pandemic funding. Dr. Ashish Jha, the White House coronavirus response coordinator, told reporters he’s worried about what could happen later this year. “What keeps me awake at night, it is that we are going to run out of vaccines,” he said. “We’re not going to be able to have enough of the next generation of vaccines. We’re going to run out of treatments. And we’re going to run out of diagnostic tests, probably in the late fall into winter, if we end up having a significant surge of infections.”
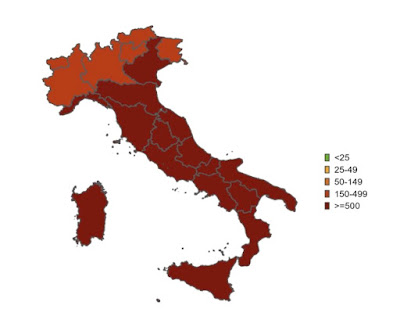 |
| New cases May 15-29 |
Italy’s map looks like the US one, though it covers two weeks rather than one:
But since deep red means 250+ weekly cases per 100,000 in Italy and only 100+ in the US, Italy is likely doing worse. We can’t know for sure given the increasing dependence in both countries on do-it-yourself tests, but I can tell you that not a day goes by without someone consulting me about a positive test, and that my secretaries tell me patients are cancelling appointments left and right because they have COVID.
The latest CDC guidelines let COVID-19 patients go back to work after 5 days and get on a plane after 10 days, with nary a swab. I’ve always had serious doubts about such leniency, and they're only increasing. We’ve known for months that more than half of Omicron patients still test positive after the 5-day mark, with somewhere between 17%and 60% still shedding live virus. Now it turns out that some patients remain positive on antigen tests for more than 10 days, some as long as 14 days, a reasonable sign that they’re still infectious… The 10-day contagious period was with earlier variants; if Omicron sticks around longer that helps explain why it spreads like wildfire.
Italy was poised to eliminate all mask mandates on June 16th, but faced with huge case numbers they’ve had the good sense to back off a mite, requiring KN-95 masks on buses, subways, and trains through September. Not on planes, because ventilation in the air has been markedly improved. But unfortunately they’re insisting on letting audiences at indoor shows and sporting events sit for hours unmasked and undistanced.
Herd immunity: fantasy and reality
 |
| Great Barrington Declaration authors meeting with Trump's HHS Secretary Alex Azar |
The let-her-rip mirage of herd immunity from natural infection turns out to have been even more deeply embedded in the Trump White House than we knew. Remember the Great Barrington Declaration? The one saying everybody except the aged and the frail should go out and get COVID-19 on purpose? It's been revealed that its authors met twice with the Trump pandemic team in the White House while the President was critically ill in the hospital. And that they had a meeting with the Man himself in August 2020, kept secret for more than a year.
But how about real herd immunity, the kind that comes when so many people are immune that a germ can’t find anybody to infect? Anthony Fauci suggested early in the pandemic that we might reach that point when 70% of the population had been vaccinated. He admitted later this was a deliberately overoptimistic underestimate, updated that estimate to 80%, and hinted that 90% might be even more realistic. Now, though, he and two colleagues are arguingthat the herd immunity concept might never apply to COVID-19. Human populations can have herd immunity against polio, smallpox, and measles because those viruses meet two criteria: only one form of the virus exists stably over time, and both vaccination and natural infection give long-lasting or even lifelong immunity. SARS-CoV-2, alas, flunks (or wins) on both counts. It’s been mutating like mad starting a year after it first appeared, vaccine efficacy starts falling off within a few months, and infection is less protective than had been hoped – especially against different variants.
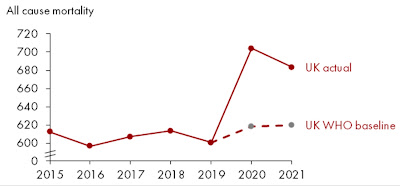
Wonking out about excess mortality
But there is an oddity buried in the WHO figures: Germany’s excess mortality is estimated as higher than the UK’s, much higher than the French, and nearly at Italian and American levels. This is very different from the figures – above – at the Our World In Data website that is my pandemic Bible.
There is an explanation, as a kindly OWID statistician informed me:
“The WHO estimates higher excess mortality in Germany because they estimate a lower baseline number of expected deaths for 2020–2021 (had the pandemic not occurred). This gives higher excess deaths, even when using the same numbers for reported (actual) deaths, because excess deaths = reported deaths – expected deaths. This Twitter thread explains a bit more what I mean.”
That fascinating Twitter thread features these very different-looking curves:
The German one sure looks suspicious, as though OWID was cheating when it gave Germany a good report card. But Germany has reported very few COVID-19 deaths despite using explicitly inclusive criteria: “Germany's case numbers include those who died ‘of’ COVID-19, and those who died ‘with’ the disease.” This suggested to me that the WHO might be wrong after all, with Germany really having low excess mortality during the pandemic.
In fact!!! The WHO received so much flak about their German numbers that it has had to eat humble pie, recalculate, and admit it messed up. Its new estimate for excess mortality in Germany is a whopping 37% lower than the original one.
Editorial chicanery
 |
| Russell Blaylock and his kooky book |
In what in medical publishing amounts to an astonishing abuse of power, the notorious retired neurosurgeon Russell L. Blaylock, Assistant Editor-In-Chief of a weird journal called Surgical Neurology International, has published a bizarre rant about COVID-19 as an editorial in his journal, often citing papers that directly contradict his conclusions (see this withering Twitter thread). Here goes:
- The virus was “engineered” to “deliberately kill and cripple millions.”
- Natural immunity is lifelong
- The media is under the control of those benefitting from this “pandemic”
- “Federal medical bureaucracies (CDC, FDA, NIAID, NIH, etc) did all in their power to prevent life- saving early treatments” because “Letting patients deteriorate to the point they needed hospitalization, meant big money for all hospitals”
- Masking, lockdowns, even testing, are “of little or no use”
- Hydroxychloroquine and ivermectin are “life-saving”
- mRNA vaccines are “essentially untested”
- Vaccines cause cancer
- Vaccines alter your DNA
- Vaccines have killed hundreds of thousands
- Vaccines spread “deadly nano-lipid carriers” throughout the body
- “Special deadly lots (batches) of these vaccines are mixed with the mass of other Covid-19 vaccines”